The robotic revolution: The future of orthopaedic surgery
By Nick Clement
Consultant Orthoaedic Surgeon, Royal Infirmary of Edinburgh, NHS Lothian Trust
Trauma and orthopaedic surgery have long been at the forefront of medical innovation, striving to enhance patient outcomes and surgical techniques. The emergence of robotic technology has introduced a new era in this field, promising unprecedented precision. The NHS seems to be on the brink of a tsunami of technological advancement that is has never seem before, with the aim of improving patient outcomes and optimising the ever-limited available resource. The integration of robots in various orthopaedic procedures would seem inevitable as technology advances but their impact on surgical outcomes and the broader implications for healthcare systems such as the NHS are not clear.
The ROBODOC was one of the original robotic systems available for hip and knee arthroplasty surgery in the early 1990s. Several systems have since become commercially available building on the principles learned from navigated surgery. The key difference being the control mechanisms used to increase the level of precision, with placement of the cutting jig or performing the cutting of the bone depending on the system used. The Robotic arm Interactive Orthopedic (RIO) MAKO (Stryker) system is the principal robot in the arthroplasty market currently. There are however an increasing number of alternative robots becoming available. However, each robotic system has its own methodology and although there may be similarities each system should be assessed and reported independently. Table 1 illustrates a method for classifying and categorising robots used in arthroplasty surgery according to the method of control (surgeon or robot), whether pre-operative imaging is required (image-based or image-less) and whether the robot cuts the bone directly or not (direct or indirect cutting).
Table 1: Classification of robotic-assisted surgical systems according to control (grey), need for pre-operative imaging (blue), and whether the robot is used to make the bone cuts or not (yellow).
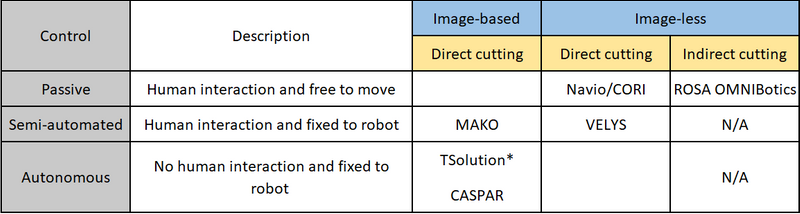
*Originally called ROBODOC, N/A not applicable
There are broadly three types of methods of control of robots available: passive, semi-active and active. Passive robotic-assistance is where the surgeon has control of the saw/burr and it is not attached to a robotic arm. A semi-active or semi-autonomous system is one where the robot aligns and controls the saw/burr and requires the surgeon to initiate the bone cut. A fully-active or autonomous system is one that does not require any input from the surgeon to perform the bone cuts. Robotic systems also vary in their navigation and registration of the patient’s anatomy and limb alignment, with some using image-based methods (plain radiographs, CT or MRI scans) which aids pre-operative planning and specified landmarks are mapped intra-operatively to match the image to the patient’s anatomy. Whereas image-less systems use surface mapping techniques based on established navigation systems with intra-operative mapping of joint line and establishing limb alignment. Some systems also work within a defined haptic boundary which is a set area beyond which the system will not cut or will shut down when the cutting blade/burr reaches this limit which has been shown to result in less soft tissue disruption.
Much of the literature reports the outcome of the MAKO (Stryker) robot. The learning curve is short, being reported to be around 10 cases for efficiency and optimal surgical time, but the learning time for precision is zero. Multiple studies have shown that the MAKO robot is more precise in implant placement than manually performed surgery for partial and total knee arthroplasty and total hip arthroplasty. This improved accuracy has been associated with a greater early survivorship of partial knee replacement when compared to manually performed surgery. Whether there is a survival benefit for robotically-assisted total knee and hip is not yet established. Some studies have shown a functional benefit of robotically-assisted surgery when compared to manually performed surgery, but whether these benefits are clinically significant again is not clear. Although some studies and reviews have shown a significant benefit the difference is often less than the minimally clinical important difference (MCID), being a change in the score that the patient does not recognise the benefit. However, it could be argued that such a difference may not be possible using currently available patient reported outcome measures (PROM). For example, the MCID in the Oxford score is 5-points and the mean change after a total knee arthroplasty is 15 points. Therefore, to show a MCID difference robotic knee arthroplasty would need to achieve a mean improvement of 20 points some 33% better than a standard manual knee arthroplasty. This improvement may be more than technology can currently offer. If the benefits of robotic surgery are to be recognised then a different approach to powering studies to a smaller effect size or a new PROM that is more sensitive to change may be needed.
One of the limitations of robotic surgery is that we now have a tool that place an implant more precisely to within plus or minus one degree but the optimal target for each patient is not yet known. Traditionally the aim was to mechanically align the total knee arthroplasty, with the purpose to try and limit the outliers to reduce the risk of revision. The robot now enables the surgeon to position the implant far more precisely, but the optimal alignment strategy is not established. Accuracy and precision are different. For example, when shooting arrows at a target if all are very close together, but they are consistently off-centre (not hitting the bullseye), they are precise but not accurate. If the arrows cluster more loosely but are centred around the bullseye, they are accurate but not precise. Precision relates to the repeatability or reproducibility of measurements, while accuracy concerns the closeness of measurements to the true value. Therefore, the optimal implant alignment needs to be defined which has led to the concept of kinematic alignment, being patient specific alignment according to their native joint line with true measured resection. However, there are some concerns when matching alignment to those patients that have varus/valgus joint lines out with plus or minus three degrees, which has led to the concept of restricted kinematic alignment. Doubtless as technology advances and increasing evidence emerges at some point in the future some artificial intelligence program will be able to define the optimal implant alignment for each patient according to their demographics and joint alignment and be able to predict their functional outcome, satisfaction and implant survival. However, robotic-assisted surgery will be needed to precisely hit this target.
The question is often asked what if the robot fails during a procedure? The simple answer for those surgeons practicing currently would be to convert to a traditional manual procedure as most would be comfortable to do this due to exposure during their training. How will this effect surgeons of the future who may get less exposure to manual surgery and may not be as competent? The analogy to airline pilots who undertake a specified number of manual take offs and landings each year to retain their skills may be needed in orthopaedics. The question would then be which patients could have a manual hip/knee arthroplasty that would not result in a worse outcome for that patient?
Finally, the current major limitation of robotic-assisted surgery is the financial cost, not only for the robot but the additional consumables needed in theatre, increased theatre time and for those image-based systems the pre-operative radiographic investigations. Whether these increased upfront costs results in cost savings form improved implant survival and PROMS in the longer term remains to be recognised.
In conclusion, adoption of robotic assisted surgery in orthopaedics would seem to be inevitable for the NHS, but whether this is currently cost effective and results in clinically significant benefit is not clear. The increased precision will likely result in enhanced implant survival due the benefits of improved implant alignment. The current definitions of what is a clinical important change may however need to be adjusted to recognise the potential clinical benefits. The next stage of the robotics evolution will likely be the adoption of artificial intelligence to use patient factors and native joint alignment to give the surgeon a patient specific target that they can repeatedly hit using robotic-assisted surgery: achieving both precision and accuracy.
Robotics in orthopaedics - Guidance documentsThe BOA and the Royal College of Surgeons of England and the Royal College of Surgeons of Edinburgh have produced a series of guidance documents to provide a practical tool kit for hospitals when setting up a new MSK robotic surgical service. A guide for patients has also been produced. View the guidance documents here. |
