Is our personal protective equipment (PPE) actually fit for purpose?
By Eilís Fitzgerald, Patrick McCabe and Eshwar Murthy
Department of Orthopaedics, Sligo University Hospital, Sligo, Ireland
Corresponding author e-mail: [email protected]
Published 15 June 2020
Introduction
The rapid emergence and worldwide spread of COVID-19 resulted in a surge in global demand and subsequent dearth of Personal Protective Equipment (PPE) for healthcare workers1. This is of significant concern due to the high risk of transmission of the virus amongst healthcare-workers due to the nature of close contact with patients2. In response to this dependence and subsequent shortage, many health care settings have been forced to procure PPE from various sources in multiple formats under a more urgent procurement pathway than ever seen before1. This is resulting in a significant heterogeneity in the equipment provided with many products from multiple manufacturing companies going into practice daily. Furthermore, the WHO guideline on PPE recommends a need for various levels of PPE depending on the particular clinical scenario in which the healthcare worker is being exposed3. This all combines to result in a motley menu of PPE across the healthcare setting. Orthopaedic practice involves close contact with patients often during aerosol generating procedures but the risk of transmission is not clearly known for orthopaedic procedures in particular4. Our study aimed to look at the various forms of PPE available and to assess the permeability of the different products to both droplet particles and aerosolised particles using a fluorescent solution and thus the risk to orthopaedic surgeons and other healthcare workers while caring for their patients.
Methods
We audited and assembled all the forms of PPE available to us in our healthcare facility (a level 3 university affiliated teaching hospital). We prepared a Fluorescein ultraviolet solution using 10ml Fluorescein stain in 5L of water. This was placed into a plastic pump spray bottle with finger pump dispersal mechanism for uniformity of solution dispersal, (Figure 1). The bottle was placed one metre and again at two metres from the subject at face level and one pump of the fluorescent solution was sprayed towards the subject wearing the various combinations of PPE. We then repeated the above tests using the fluorescent aerosolised spray for a three second spray duration.

Under ultra-violet (UV) light the PPE itself was examined and any penetration of the fluorescent solution through the material was recorded, (Figure 2). Equipment was initially graded on a pass or fail system determined by either the absence or presence of visible UV residue on the inside of the material respectively. The number of UV droplets visible was also counted.

The subjects face was examined on removal of the PPE was performed in line with the national doffing protocol. Once again a pass/fail system was used to determine if fluorescent droplets were visible on the face in areas which should have been covered by the PPE and again the number of visible droplets was counted.
The areas of skin not covered by the PPE and the subjects hands were also assessed post doffing for any residue which may indicate a risk of cross contamination during or post doffing.
Clinical images of the PPE and the subjects face and hands were taken throughout the testing and these images were then used to perform inter-rater reliability testing of the above methods between three different assessors, (Figure 3 a-e).

Figure 3a: Medical mask
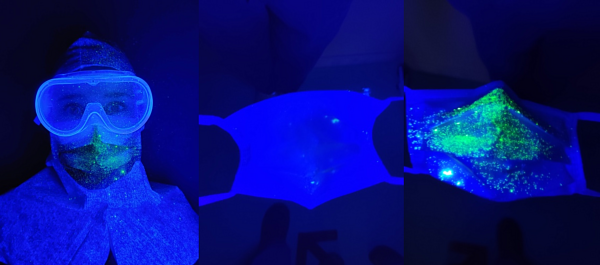
Figure 3b: Surgical mask
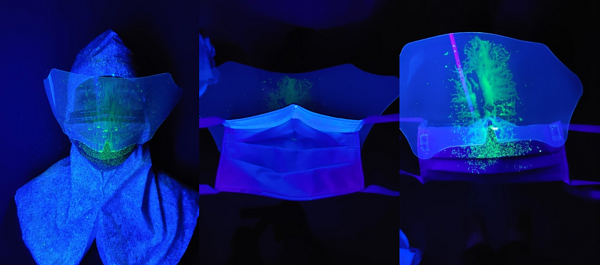
Figure 3c: Mask-visor combination
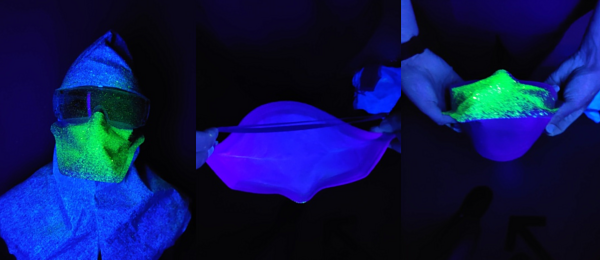
Figure 3d: FFP2/FFP3

Figure 3e: Face shield
Results
Five mask types were assessed in this trial – regularly stocked surgical mask stock, COVID-19 emergency stock ‘medical mask’, surgical mask with attached visor, FFP2 and FFP3 masks, (Figure 4).
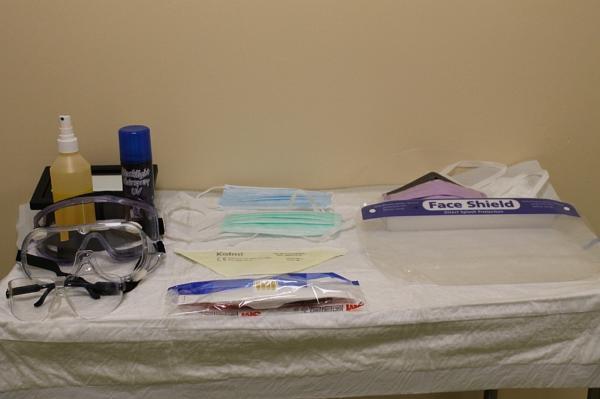
Figure 4: Table set-up
Five forms of visor were also tested – Sealed rubber goggles, non-sealed rubber goggles, plastic safety glasses, face shield and the surgical mask – visor combination product.
No material showed penetrance through the material onto the subjects face and there was no cross-contamination seen on the subjects hands post doffing for any procedure.
The FFP2/FFP3 masks showed no penetrance of either the droplet or aerosolised solution through the material. They did however have a large volume of particulate visible on the upper-outer portion of the mask highlighting the importance of proper doffing procedures, (Figure 5). All surgical masks showed penetrance of the UV droplet but not the aerosol. The COVID-19 emergency stock masks showed greater penetrance than the routine stock surgical masks, (Figure 4). The mask-visor combination product showed the least penetrance of all the surgical masks. There was no penetrance through any material when droplet particulate was sprayed from a two metre distance again in line with current guidelines and recommended social distancing guidelines.
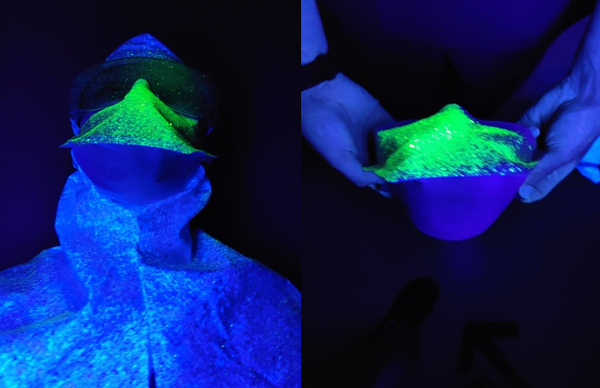
Figure 5
The subjects face outside of what was covered by PPE showed significant contamination in all close contact (1m) droplet and aerosol tests, except when using the face shield. This led us to repeat the testing process using a fresh surgical hood between tests to avoid cross contamination between tests, (see Figure 6). This was an unexpected discovery as the amount of contamination was significant in areas not covered by the PPE, (Figure 6) and is concerning from an infection control perspective.
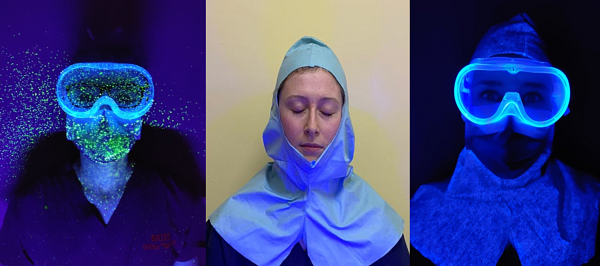
Figure 6
The face shield gave the most protection to the subjects face itself but it did allow both aerosol and droplets to penetrate between the shield and the head band due to a gap in the device proximally. This resulted in residue being visible across the forehead and areas close to the subjects eyes which is a potential route of infection. The other three eye protection devices performed similarly with no penetrance, (Table 1).
|
|
|
COVID-19 “Medical Mask” |
Surgical Mask |
Mask-visor combination |
FFP2 |
FFP3 |
Sealed goggles |
Un-sealed goggles |
Safety glasses |
Face-shield |
|
Droplet – 1m |
Penetrance Y/N |
Yes |
Yes |
Yes |
No |
No |
No |
No |
No |
Yes |
|
|
Amount of penetrance |
Significant (>5) |
Moderate (2-5) |
Minimal (<2) |
|
|
|
|
|
Minimal |
|
Droplet – 2m |
Penetrance Y/N |
No |
No |
No |
No |
No |
No |
No |
No |
No |
|
|
Amount of Penetrance |
|
|
|
|
|
|
|
|
|
|
Aerosol 1m |
Penetrance Y/N |
Yes |
Yes |
Yes |
No |
No |
No |
No |
No |
No |
|
|
Amount of penetrance |
Significant (>5) |
Modertate (2-5) |
Minimal (<2) |
|
|
|
|
|
|
|
Aerosol 2m |
Penetrance Y/N |
No |
No |
No |
No |
No |
No |
No |
No |
No |
|
|
Amount of Penetrance |
|
|
|
|
|
|
|
|
|
Discussion
PPE is critical to protecting frontline healthcare workers, particularly during this global pandemic. COVID-19 can be spread via droplets up to a distance of two metres, but also via aerosol once an aerosol-generating procedure (AGP) has been performed5. AGPs are commonplace in the operating theatre, both from an anaesthetic perspective, at induction of general anaesthesia, but also during the operative procedure itself, e.g. the use of high speed drills in dental6 or otorhinolaryngological procedures7. It is not definitively known whether AGP’s performed during orthopaedic operations pose a significant risk4. Therefore it is crucial that our staff are appropriately protected when exposed to this potentially high risk environment.
Given the multiplicity of COVID-19’s mechanism of spread, an assortment of PPE has been provided to our staff and recommended by our governing bodies depending on the level of risk or exposure to the hazard. Our study showed that all PPE does not offer the same level of protection and as such a clear understanding of the specific PPE guidelines is crucial in the protection of orthopaedic surgeons and associated healthcare workers. The level of penetrance we demonstrated generally coincided with the level of PPE, which is based on the level of situational exposure, apart from the face shield. The face shield is currently recommended for use during AGPs but our study demonstrated that it allowed particulate to land close to the orbital mucous membranes, thus risking viral transmission. These shields are being mass produced by many non-medical manufacturing companies in response to the global PPE shortage, but the version supplied to our institution featured a significant design flaw, with the presence of a gap between the shield itself and the subjects face. The emergency stock available in our hospital was deemed the lowest quality in testing. The defect in the face shield and the high level of penetrance of particulate in the ‘COVID-19 stock’ surgical masks highlight how poor quality PPE could put the user at considerable risk of contamination. This highlights the need for vigilant quality control in both the production and procurement of all forms of PPE in a time of pandemic.
Conclusion
Our experiment illustrated a novel method of testing efficacy of equipment in a time of pandemic when multiple manufacturers, materials and formats of PPE are in use. The surgical masks demonstrated some level of material penetrance during each individual droplet trial and as such should only be used in what are deemed low risk environments, which is in line with current guidelines. Unfortunately, the PPE face shield allowed for transmission of a considerable volume of particles onto the testers face bringing to question its efficacy during droplet contact or aerosol generating procedures. Proper donning and doffing in accordance with protocol is critical to prevent potential cross contamination and our study also highlights the protection a simple surgical hood affords healthcare workers during high risk procedures. Our results support the guidelines of the Health Service Executive (HSE) and World Health Organisation (WHO).
References
- Jessop ZM, Dobbs TD, Ali SR, Combellack E, Clancy R, Ibrahim N, et al. Personal Protective Equipment (PPE) for Surgeons during COVID-19 Pandemic: A Systematic Review of Availability, Usage, and Rationing. Br J Surg. 2020; May 12. [Epub ahead of print].
- Ağalar C, Öztürk Engin D. Protective measures for COVID-19 for healthcare providers and laboratory personnel. Turk J Med Sci. 2020;50(SI-1):578-84.
- World Health Organization (2020). Rational use of personal protective equipment for coronavirus disease (COVID-19) and considerations during severe shortages - Interim guidance - 6 April 2020. Available at: https://apps.who.int/iris/bitstream/handle/10665/331695/WHO-2019-nCov-IPC_PPE_use-2020.3-eng.pdf.
- Basso T, Dale H, Langvatn H, Lønne G, Skråmm I, Westberg M, et al. Virus transmission during orthopedic surgery on patients with COVID-19 - a brief narrative review. Acta Orthop. 2020 May 14. [Epub ahead of print].
- Wu YC, Chen CS, Chan YJ. The outbreak of COVID-19: An overview. J Chin Med Assoc. 2020;83(3):217‐20.
- Odeh ND, Babkair H, Abu-Hammad S, Borzangy S, Abu-Hammad A, Abu-Hammad O. COVID-19: Present and Future Challenges for Dental Practice. Int J Environ Res Public Health. 2020;17(9):E3151.
- Mick P, Murphy R. Aerosol-generating otolaryngology procedures and the need for enhanced PPE during the COVID-19 pandemic: a literature review. J Otolaryngol Head Neck Surg. 2020;49(1):29.
